Pregnancy Registries: What We’re Learning About Medication Safety
When a woman takes a medication during pregnancy, no one knows for sure how it will affect the baby-not even her doctor. Clinical trials don’t include pregnant women. That’s not because they’re being ignored-it’s because it’s unethical to test new drugs on a developing fetus. So how do we find out if a medicine is safe? The answer lies in something most people have never heard of: pregnancy registries.
What Are Pregnancy Registries, Really?
Pregnancy registries aren’t databases you can browse online. They’re active, ongoing research projects that track women who take specific medications while pregnant. These registries collect detailed information-what drug was taken, when, how much, and whether the baby was born healthy or had complications. The goal? To spot patterns that might mean a drug could cause birth defects or other problems. These programs started after the thalidomide disaster in the 1960s, when thousands of babies were born with severe limb deformities because their mothers took a drug thought to be safe. Since then, regulators like the FDA and EMA have required pharmaceutical companies to set up registries for drugs likely to be used by women of childbearing age-especially biologics, psychiatric meds, and antiseizure drugs. Unlike passive systems that wait for doctors or patients to report side effects, registries reach out to women directly. They enroll participants as soon as possible after a woman finds out she’s pregnant and is taking a specific medication. Then they follow her through birth-and sometimes beyond, tracking the baby’s development for up to a year.Why Do We Need Them?
About 80% of pregnant women in the U.S. take at least one medication during pregnancy. Some are for chronic conditions like depression, epilepsy, or autoimmune diseases. Others are for short-term issues like infections or nausea. But for most of these drugs, we simply don’t have good safety data. Take antidepressants. Many women worry about taking SSRIs like sertraline or fluoxetine while pregnant. Some studies suggest a small increased risk of heart defects. Others say there’s no real danger. Without registries, we’d be stuck guessing. But registries like the National Pregnancy Registry for Psychiatric Medications have tracked over 3,000 pregnancies so far. Their data shows that while some drugs carry tiny risks, others appear to be very low risk. That’s not just reassuring-it’s life-changing for women deciding whether to continue treatment. The same goes for biologics used to treat rheumatoid arthritis or Crohn’s disease. These powerful drugs suppress the immune system. Before registries, doctors often told women to stop them before getting pregnant, even if it meant their disease flared up. Now, registry data shows many of these drugs can be safely continued through pregnancy, helping moms stay healthy without harming their babies.How Do They Work?
Enrollment is voluntary. Women are usually contacted by their OB-GYN, pharmacist, or through patient advocacy groups like MotherToBaby. Once enrolled, they’re asked to fill out surveys, share medical records, and sometimes have follow-up calls or visits. The data collected includes:- Exact medication name, dose, and timing of exposure
- Other medications taken during pregnancy
- Maternal health conditions (diabetes, hypertension, mental health disorders)
- Smoking, alcohol, or drug use
- Pregnancy outcomes: miscarriage, preterm birth, stillbirth
- Baby’s birth weight, gestational age, and major birth defects
- Developmental milestones up to 12 months after birth
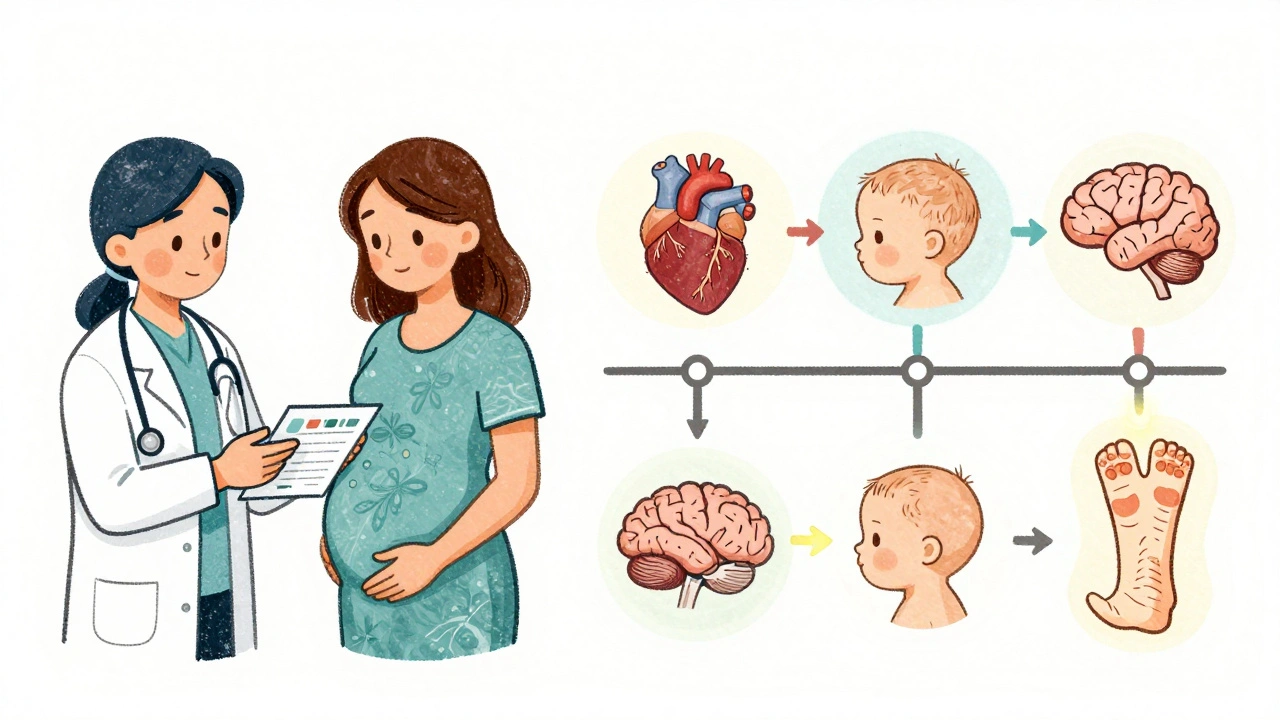
What Can They Tell Us-and What Can’t They?
Registries are powerful, but they’re not magic. They’re great at spotting major risks-like a drug causing a clear pattern of heart defects. But they struggle with small risks. If a drug increases the chance of a rare defect from 1 in 1,000 to 2 in 1,000, you’d need over 1,200 exposed pregnancies to be sure. Most registries only enroll a few hundred women. They also can’t prove a drug is completely safe. They can only say it doesn’t appear to cause major harm. As one expert put it: “Registries can’t prove safety. But they can give reassurance.” Another limitation? Selection bias. Women who volunteer for registries are often more informed, more anxious, or more motivated to help science. They might be more likely to report every symptom or detail. That doesn’t mean the data is wrong-it just means it’s not a perfect mirror of the general population. And while registries are better than relying on hospital records or insurance claims (which often miss exact drug details), they still can’t control for everything. A woman with severe depression might be more likely to have a preterm baby-not because of the medication, but because of the illness itself. That’s why newer registries now compare women taking the drug to women with the same condition who aren’t taking it. That’s called a “matched comparator group.” It’s a big step forward.Who’s Running These Registries?
In the U.S., there are over 80 active pregnancy registries. Most are run by pharmaceutical companies as part of their regulatory obligations. But many are managed by academic medical centers. The National Pregnancy Registry for Psychiatric Medications, for example, is run by Massachusetts General Hospital. Others are run by universities or nonprofit groups like MotherToBaby, which partners with drug makers and provides free counseling to participating families. The biggest focus areas? Psychiatric medications (12 registries), antiepileptics (8), and biologics for autoimmune diseases (15). That’s because these are the drugs women are most likely to need long-term-and the ones with the most uncertainty around safety. In 2024, the psychiatric registry expanded to include 18 new medications, bringing its total to 45. That’s a lot of data being gathered in real time.Challenges and Real-Life Hurdles
Despite their value, registries face big problems. Only 15-20% of eligible women enroll. Why? Many are scared. They worry the registry will pressure them to stop their meds. Others think they’ll get immediate answers-when in reality, it takes years to analyze data. Some don’t want to share personal health info. Others just don’t have time. Retention is another issue. About 20-30% of women drop out before giving birth. Life gets busy. Appointments get missed. Fear takes over. Registries have to work hard to stay in touch-through texts, phone calls, even home visits in some cases. Cost is a barrier too. Running a registry can cost between $500,000 and $2 million a year. That’s why many are shrinking or closing. The FDA and EMA are pushing for more coordination-like the Pregnancy Safety Research Network launched in 2022-to share resources and avoid duplication.
What’s Next?
The future of pregnancy safety monitoring isn’t just registries. It’s a mix. Registries will still be used to detect signals-but then, those signals will be checked against massive health databases from insurance claims, electronic records, and national birth registries. That’s the “tiered approach” experts now recommend. Some registries are starting to link directly to electronic health records. Imagine a woman takes a new antidepressant. Her doctor enters it into the system. The registry automatically pulls in her lab results, prescriptions, and delivery records. That cuts down on paperwork and improves accuracy. The goal? To move from “We don’t know” to “We know enough to help.” For women with chronic conditions, that means fewer forced medication stops. Fewer anxiety-filled nights. More confidence to carry a healthy pregnancy.What Should You Do If You’re Pregnant and Taking Medication?
Don’t stop your meds without talking to your doctor. But do ask: “Is there a pregnancy registry for this drug?” If there is, consider joining. You’re not just protecting your baby-you’re helping other women in the future. If you’re not sure, call MotherToBaby at 1-866-626-6847. They’re free, confidential, and staffed by specialists who know the latest registry data. You don’t have to enroll to get advice. And if you’re a healthcare provider? Talk to your patients about registries. Don’t assume they know they exist. Many don’t. A simple question-“Have you heard about pregnancy safety studies?”-can open the door to better decisions and better outcomes.Final Thoughts
Pregnancy registries aren’t flashy. They don’t make headlines. But they’re quietly saving lives. For every woman who’s been told to “just avoid all meds,” these registries are giving her real data. For every doctor who’s been guessing, they’re offering clarity. For every baby born healthy despite exposure to a medication, there’s a registry that helped prove it was safe. They’re not perfect. But they’re the best tool we have right now. And they’re getting better.Are pregnancy registries safe to join?
Yes. Participation is completely voluntary and confidential. Registries follow strict privacy rules, and your personal health data is never shared with drug companies or insurers. You can withdraw at any time. Many women say they feel empowered knowing they’re helping future moms make safer choices.
Can a registry tell me if my medication is safe right now?
Not immediately. Registries collect data over years, not days. You won’t get a quick answer like “This drug is safe.” But you will get access to the latest available evidence. If the registry has data on your drug, you’ll learn what’s known so far-and what’s still uncertain. That’s better than guessing.
Do I need to be on a prescription to join a registry?
Yes. Registries only track women taking specific, approved medications-usually those with known or potential risks. Over-the-counter drugs, supplements, or herbal remedies are rarely included unless they’re part of a specific study. If you’re unsure whether your drug is tracked, ask your provider or contact MotherToBaby.
How long do registries follow children after birth?
Most track babies for at least 12 months, focusing on growth and major developmental milestones. Some, especially those studying psychiatric or neurological drugs, follow children for several years to check for learning delays, autism spectrum traits, or behavioral issues. This long-term tracking is what makes registries so valuable-they don’t just look at birth defects, but how the child develops over time.
Are pregnancy registries only in the U.S.?
No. Similar programs exist in Europe, Canada, Australia, and parts of Asia. The European Medicines Agency requires registries for many new drugs, and many are run through national health systems. Global collaboration is growing, with data-sharing efforts between the U.S., EU, and WHO to improve safety insights across borders.


15 Comments
Aileen Ferris
December 12 2025i heard these registries are just a way for big pharma to avoid lawsuits lol. like sure, they "track" stuff but if your kid has a defect, good luck getting them to admit it was the drug. also, why do they only track prescriptions? what about the weed and energy drinks? 🤷♀️
Sarah Clifford
December 13 2025i just took zoloft while pregnant and my kid is now a genius who speaks 4 languages and plays the violin. so yeah, it's fine. also, i didn't even know these registries existed until now and i'm mad i didn't sign up. i could've been a hero for science.
Ben Greening
December 13 2025The methodology of pregnancy registries represents one of the most ethically sound approaches to pharmacovigilance in obstetrics. While limitations such as selection bias and sample size are acknowledged, the longitudinal nature of data collection provides a level of evidence unattainable through passive surveillance systems.
Mia Kingsley
December 14 2025okay but like... if you're taking antidepressants during pregnancy and your baby turns out fine, does that mean it was safe? or did you just get lucky? and why do they only follow kids for a year? what about when they start school and can't focus or start crying for no reason? someone needs to track this longer. also, i think they should track dads too. what if the dad was on meds before conception? no one talks about that.
Aman deep
December 14 2025this is actually beautiful. i'm from india and we don't have much of this here. my sister took medicine for her epilepsy during pregnancy and everyone told her to stop. she didn't. her son is 5 now, brilliant, healthy, loves soccer. if there was a registry like this back then, she wouldn't have felt so alone. thank you for sharing this. we need more of this in the global south.
Sylvia Frenzel
December 16 2025another american medical industry scam. we don't need more data collection. we need less drugs in pregnant women. period. if you need antidepressants to get through pregnancy, maybe you shouldn't be pregnant. also, why is this even allowed? europe has stricter rules. we're falling behind.
Regan Mears
December 17 2025I really appreciate how this post breaks down both the power and the limits of registries. It’s not perfect, but it’s the best tool we have-and it’s evolving. The matched comparator groups? That’s huge. It means we’re finally starting to separate the effect of the illness from the effect of the drug. That’s science, done right.
Vivian Amadi
December 18 2025this is why women are being manipulated. they’re told to "join" and "help future moms" but no one tells them it takes 5 years to get results. meanwhile, their anxiety spikes, their insurance drops them, and their OB won’t answer their texts. registries are a distraction from real healthcare reform.
matthew dendle
December 19 2025so let me get this straight... you take a drug that could kill your baby but you join a registry so some pharma company can say "we tried"? lol. also why do they spell it registry with an s? its regestry in my book
Jean Claude de La Ronde
December 19 2025if we're tracking every pill a pregnant woman takes, are we also tracking the existential dread? the sleepless nights? the fear of becoming a mother in a world that doesn't care? maybe the real safety signal isn't in the birth defects-it's in the silence after the baby cries for the first time.
Jim Irish
December 19 2025This is vital work. Many countries lack even basic prenatal pharmacovigilance. The U.S. system isn't perfect but it's a model. We need global standards, not just American ones.
Jimmy Kärnfeldt
December 21 2025i just found out my cousin joined a registry for her anxiety med and it made her feel so much less alone. like, she wasn't just some number in a chart-she was part of something bigger. and now she gets updates every few months. it's small, but it matters. thank you for writing this.
Ariel Nichole
December 23 2025I'm so glad this exists. My mom took a seizure med during my pregnancy and I'm turning 28 next month. No issues. I hope registries keep growing so more people like me can know we're okay.
Stephanie Maillet
December 24 2025I love how this isn't just about drugs-it's about trust. About women being treated like rational, capable people who can make informed choices... even when the science is messy. That’s the real win here.
David Palmer
December 25 2025so basically... we're asking women to be lab rats for big pharma? and then patting ourselves on the back like we're heroes? nah. i'd rather just not take any meds. my kid's fine. problem solved.