Generic Drug Acceptance: Why It Matters and What You Need to Know
When you hear generic drug acceptance, the willingness of patients, doctors, and insurers to use lower-cost versions of brand-name medications. Also known as drug substitution, it’s the quiet engine behind most prescription savings in the U.S. and beyond. But acceptance doesn’t mean automatic trust. Many people worry that generics are weaker, less safe, or made in sketchy labs. The truth? Most generics work just like the brand name—but not all are treated the same by regulators, pharmacies, or insurers.
One big reason for confusion is the difference between authorized generics, the exact same drug as the brand, made by the brand company and sold under a different label and regular generics, which are made by other companies using the same formula. Authorized generics are identical in every way—same inactive ingredients, same factory, same packaging. But they’re rarely labeled as such on your receipt. Then there’s mandatory substitution, laws that force pharmacies to swap brand drugs for generics unless the doctor says no. These rules vary wildly: in some states, you get a generic unless you push back. In others, you have to ask for it. And in the EU, substitution rules are tied to national health systems, not individual pharmacies.
Insurers push hard for generics because they cut costs by up to 95%. But that doesn’t mean they always get the best option. FDA drug list, the official database of approved generic drugs and their manufacturers is public—but it’s messy. It doesn’t tell you which version a pharmacy actually stocks, or if it’s an authorized generic. Many patients get a different generic every refill, and sometimes those switches cause side effects—even when the active ingredient is the same. That’s why some doctors avoid generics for drugs like levothyroxine or seizure meds, where tiny changes in absorption can make a big difference.
And here’s the hidden problem: when a generic fails, who’s to blame? The pharmacist? The manufacturer? The doctor who prescribed it? Because generic makers have legal immunity in many cases, physicians are increasingly on the hook if a patient has a bad reaction. That’s why some doctors now write "dispense as written" on prescriptions—not to be difficult, but to avoid liability.
What you’ll find in the posts below are real stories and facts about how generic drug acceptance works—or doesn’t—in practice. You’ll learn how to spot an authorized generic, why your insurance might block a brand even if it’s safer for you, how foreign manufacturing affects quality, and what to do if a generic makes you feel worse. This isn’t theory. It’s what happens when money, law, and medicine collide—and how to protect yourself in the middle of it.
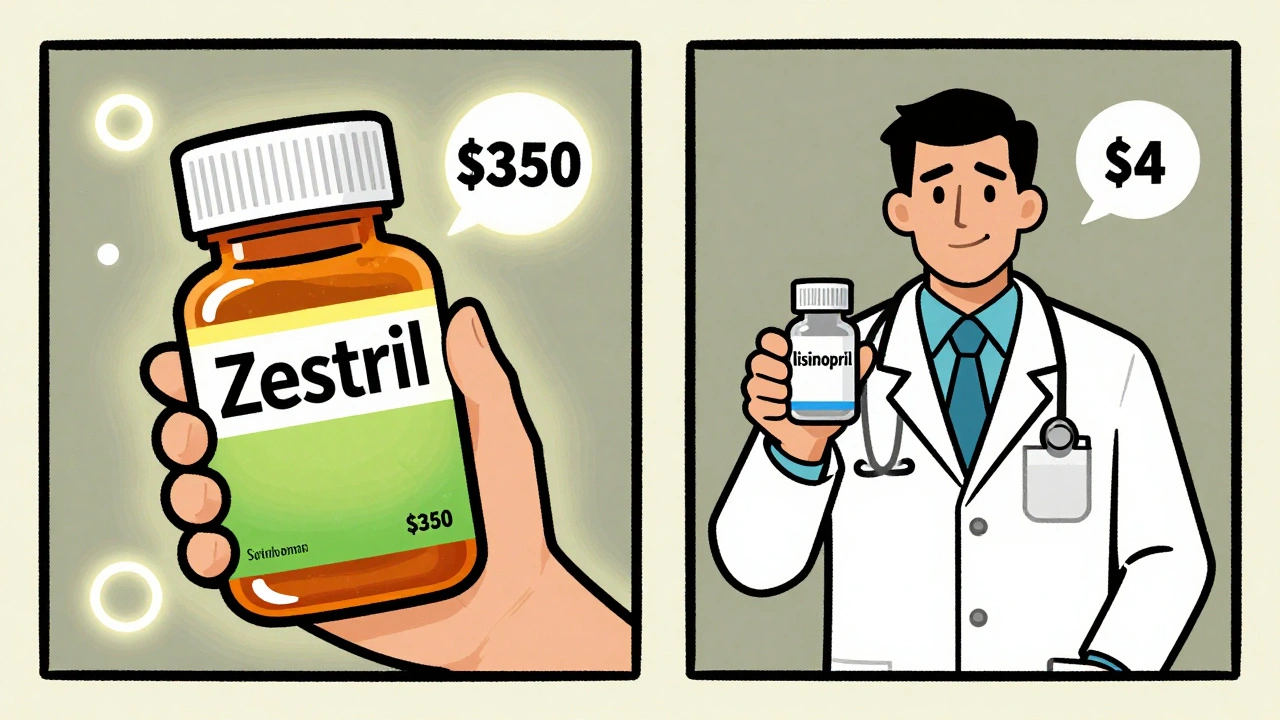
Generics work just as well as brand-name drugs - and save patients up to 90% on costs. Yet many doctors still prescribe brands, and patients refuse generics. Here’s why - and how to overcome the psychology behind the resistance.
Continue Reading





