Stability Testing Requirements: Temperature and Time Conditions for Pharmaceutical Products
When you take a pill, injection, or cream, you expect it to work exactly as it should - not weaker, not toxic, not broken down. That’s not luck. It’s the result of strict stability testing, a process that tracks how drugs change over time under real-world conditions like heat, humidity, and light. If this testing fails, the medicine could lose potency or even become dangerous. The rules for this testing aren’t made up by one company or country - they’re set by global standards, mostly from ICH Q1A(R2), and followed by the FDA, EMA, and health agencies worldwide.
What Stability Testing Actually Does
Stability testing answers one simple but critical question: How long can this drug stay safe and effective before it degrades? This isn’t about shelf life on a grocery store item. This is about life-saving medicines that must remain within tight chemical limits - often 95% to 105% of the labeled strength - for years. A 5% drop in potency might sound small, but for a cancer drug or insulin, it could mean the difference between treatment and failure.
The test doesn’t just check if the pill looks the same. It measures chemical breakdown, particle size changes, moisture absorption, and even how the drug dissolves in the body. If the active ingredient turns into something else - or if the packaging lets in too much moisture - the product fails. That’s why regulators require proof before any drug hits the market.
Temperature and Humidity: The Core Conditions
The ICH Q1A(R2) guideline, finalized in 2003 and still in force today, defines three main testing environments. These aren’t arbitrary. They’re based on real climate data and how products behave under stress.
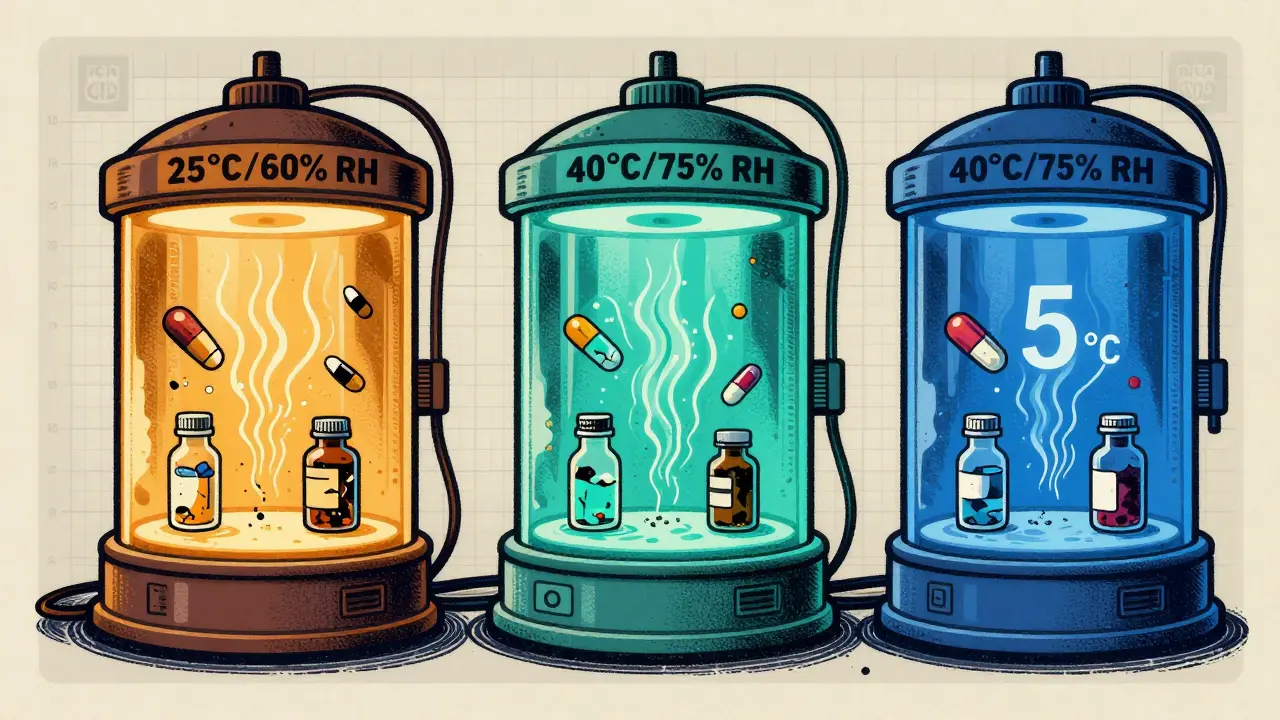
- Long-term testing: This is the real-world simulation. For most solid oral products, you store them at either 25°C ± 2°C and 60% RH ± 5% RH or 30°C ± 2°C and 65% RH ± 5% RH. The choice depends on where the product will be sold. If you’re targeting tropical markets, you use the 30°C/65% RH condition. In cooler regions, 25°C/60% RH is common. You need at least 12 months of data before you can apply for approval in the U.S. (FDA), though Europe allows 6 months under certain options.
- Accelerated testing: This is the speed test. You put the drug at 40°C ± 2°C and 75% RH ± 5% RH for 6 months. This isn’t meant to mimic real life - it’s meant to predict what will happen over years. At this level, most small-molecule drugs degrade about 4 times faster than at 25°C. That means 6 months here can roughly equal 2 years of real-time aging - but only if the degradation is predictable. For some drugs, like those that absorb water easily, this correlation breaks down.
- Intermediate testing: This is a backup. If your accelerated test shows a problem but your long-term test is at 25°C (not 30°C), you run a 6-month test at 30°C/65% RH. It’s a bridge between the two. You don’t do this unless something changes during accelerated testing.
For refrigerated products - like insulin or some biologics - the rules shift. Long-term is kept at 5°C ± 3°C, and accelerated is done at 25°C ± 2°C and 60% RH, not the usual 40°C. Why? Because freezing and thawing are bigger risks than heat for these products. A single freeze-thaw cycle can destroy a monoclonal antibody. That’s why the old 40°C rule doesn’t work here.
How Long Do You Really Need to Test?
Testing doesn’t stop after 6 or 12 months. The full timeline typically goes out to 36 months - and sometimes longer. The schedule is usually: 0, 3, 6, 9, 12, 18, 24, and 36 months. Early time points (3 and 6 months) are critical because that’s when most degradation shows up. If a drug starts falling out of spec at 6 months, you can’t wait until 12 to know.
Here’s the catch: You can’t submit a drug for approval without at least 12 months of long-term data in the U.S. That means if you start testing in January 2025, you can’t file until January 2026 - even if the accelerated data looks perfect. For a company launching a new drug, that’s a full year of waiting. Many biotechs outsource this to CROs because running these chambers costs $185,000 to $275,000 per product.
What Happens When Things Go Wrong?
Failure isn’t rare. In 2022, the FDA issued 27 warning letters specifically for stability testing problems. One case involved Teva Pharmaceuticals: their generic version of Copaxone® didn’t detect protein aggregation at 40°C. The result? A voluntary recall of 150,000 vials. Another case, at Amgen, involved a monoclonal antibody that degraded during shipping because the stability protocol didn’t account for temperature spikes in warehouses.
Even small changes matter. One pharmacist on Reddit shared how a Pfizer team lost a regulatory review because a batch showed 94.8% potency - just below the 95% lower limit. Statistically, it was a rounding error. But regulators don’t care about statistics. They care about specs. One decimal point can trigger a rejection.
Chambers also fail. A 2023 survey of 142 stability professionals found that 78% had experienced at least one temperature excursion over ±2°C during a study. One degree too hot for a week can invalidate the whole test. That’s why chambers must be qualified with IQ/OQ/PQ protocols, mapped for hot spots, and monitored with continuous data loggers. Humidity control is just as hard - especially in dry climates. Some labs install dual-loop systems to cut RH swings from ±8% down to ±3%.
Why the Rules Are Struggling to Keep Up
The ICH Q1A(R2) guidelines are 20 years old. They were built for pills and injections - not mRNA vaccines, antibody-drug conjugates, or lipid nanoparticles. These new drugs are fragile. A single freeze-thaw cycle can ruin them. Standard 40°C tests don’t predict that. In fact, a 2021 study by Dr. Lisa McLeod showed the current protocols fail for 35% of hygroscopic compounds and nearly all biologics.
Even the definition of “significant change” is vague. The guideline says it’s a change that affects safety or efficacy - but doesn’t say how much. Is a 3% drop significant? 5%? That’s left to interpretation. One regulator might accept it. Another might shut you down. That’s why companies spend months arguing with inspectors over data.
And now, with continuous manufacturing and real-time release testing, some experts - like Dr. Robert Smith - argue the whole system is outdated. If you’re testing every batch as it’s made, why wait two years to prove stability? The FDA is testing this idea now with pilot programs using process analytical technology (PAT). If it works, future drugs might need far less physical testing.

What’s Changing in 2025 and Beyond
The ICH Q1 working group is drafting a new update - Q1F - expected by late 2024. It’s meant to cover complex products like ADCs and cell therapies. That’s a big deal. Right now, there’s no official guidance for these drugs. Companies are guessing.
More companies are turning to predictive modeling. Instead of waiting 24 months, they run tests at 50°C, 60°C, even 80°C - way beyond ICH limits - and use math to extrapolate. A 2022 study found 74% of top pharmaceutical companies now use this. It can cut time-to-market by a year. But regulators are skeptical. The EMA rejected 8 model-based submissions in 2022-2023 because they didn’t trust the math.
For now, the rules stay the same. If you’re developing a drug, you still need to store it at 25°C or 30°C, run 6 months at 40°C, and wait 12 months before filing. The system is slow. It’s expensive. But it’s the only one that works globally.
What You Need to Get Started
If you’re new to stability testing, here’s the reality check:
- Start with the ICH Q1A(R2) document. Don’t skip it. It’s the bible.
- Choose your storage conditions based on your target markets. Don’t assume 25°C is enough.
- Qualify your chambers. Do temperature mapping. Document everything. One missed hotspot can cost you millions.
- Plan for delays. The 12-month wait is real. Build it into your timeline.
- Don’t underestimate humidity. Many failures come from moisture, not heat.
- Train your team. Stability testing is repetitive but requires deep understanding. A 6-9 month learning curve is normal.
The industry is worth $2.8 billion and growing. Companies like WuXi AppTec and Charles River Labs make billions running these tests for others. But behind every test is a simple goal: make sure the next pill you take won’t fail you when you need it most.
What are the standard temperature and humidity conditions for long-term stability testing?
The ICH Q1A(R2) standard allows two options: 25°C ± 2°C with 60% RH ± 5% RH, or 30°C ± 2°C with 65% RH ± 5% RH. The choice depends on the climate zone of the target market. For example, products sold in tropical regions (Zone IVa) use the 30°C/65% RH condition. The FDA requires at least 12 months of data under these conditions before approving a drug.
Why is accelerated testing done at 40°C and 75% RH?
The 40°C/75% RH condition is designed to simulate extreme environmental stress - like a drug being stored in a hot warehouse or shipped in summer. It’s not meant to reflect normal conditions. At this level, most small-molecule drugs degrade about four times faster than at 25°C, so 6 months of testing here can predict 2-3 years of real-time stability. However, this correlation doesn’t hold for hygroscopic or biologic products.
How long does stability testing take before a drug can be approved?
In the U.S., the FDA requires a minimum of 12 months of long-term stability data at the time of submission. In Europe, regulators allow either 6 or 12 months depending on the submission option chosen. Accelerated testing (6 months at 40°C/75% RH) supports the long-term data but doesn’t replace it. Full shelf-life data (24-36 months) is collected after approval.
What happens if a stability test fails?
A failed stability test can trigger regulatory action - including warning letters, product recalls, or even withdrawal of marketing authorization. For example, in 2021, Teva recalled 150,000 vials of Copaxone® after stability testing revealed aggregation issues at 40°C. Even small deviations - like a 4.8% potency drop below the 95% lower limit - can be rejected if they violate the product’s specification.
Are the current stability testing guidelines outdated?
Yes, for many modern drugs. ICH Q1A(R2) was written for traditional pills and injections, not mRNA vaccines, monoclonal antibodies, or lipid nanoparticles. These newer products degrade in ways the old tests can’t predict - like through freeze-thaw cycles or humidity swings. Experts and regulators agree the guidelines need updating. A new ICH Q1F draft is expected in late 2024 to address these gaps.
Can predictive modeling replace physical stability testing?
Some companies use predictive modeling - running tests at higher temperatures (50-80°C) and using math to forecast degradation - to speed up development. About 74% of top pharma companies now use it. But regulators like the EMA have rejected 8 model-based submissions since 2022 because they don’t trust the extrapolation. For now, physical testing is still mandatory. Modeling is a supplement, not a replacement.



14 Comments
Ben Harris
December 25 2025So basically we're paying billions to keep pills in a fancy oven for a year just so Big Pharma can charge $1000 for insulin
And if you think that's wild wait till they start testing on your kids
Winni Victor
December 26 2025I swear if one more person tells me about ICH guidelines I'm going to throw my lab coat into a incinerator and move to a cabin in the woods where the only drug is wild mint tea and a really good hammock
Zabihullah Saleh
December 27 2025You know what's wild? We treat medicine like it's a math problem when it's really a living thing. A pill isn't just molecules-it's someone's hope in a capsule. And we're measuring its death in percentages and temperature spikes. Kinda messed up when you think about it.
sagar patel
December 29 2025The 40C test is a joke for biologics. My lab ran a study where a protein degraded 90% in 72 hours at 40C but was fine at 30C. The guideline ignores biology for convenience
Jason Jasper
December 30 2025I've seen chambers fail because someone left the door open for 12 minutes. One degree off. One week. And suddenly your 12-month study is trash. No one talks about how much human error is baked into this whole system. We're not robots. We forget. We rush. And then the regulators act like we're all villains.
Rick Kimberly
January 1 2026The ICH Q1A(R2) framework remains the most robust and empirically validated system for ensuring pharmaceutical integrity across global markets. While emerging modalities present novel challenges, the foundational principles of controlled environmental exposure, statistical rigor, and longitudinal data collection remain indispensable. Any proposed modifications must be subjected to peer-reviewed validation before regulatory adoption.
Lindsay Hensel
January 2 2026I work in a lab that tests vaccines. The humidity control alone is a nightmare. We have two backup systems, two data loggers, and a guy who checks it every 90 minutes. And still, last year, a batch got rejected because of a 2% RH swing. It’s not about perfection. It’s about proving we tried everything.
Christopher King
January 2 2026They say the system works but have you seen the paperwork? Every single humidity spike gets logged in triplicate. Meanwhile, the same company that made the drug also made the chamber software. And the regulators? They get invited to fancy dinners by the CROs. This isn't science. It's a ritual. And we're all just priests in white coats chanting the same prayers for 20 years.
Terry Free
January 4 2026If your drug can't survive 40C and 75% RH for 6 months then maybe it shouldn't be a drug. Simple. Stop overcomplicating it with your fancy modeling and your biologics and your 'but it's fragile' excuses. If it breaks in a warehouse, it breaks in a warehouse. People die because of weak meds. Stop coddling the science.
Linda B.
January 5 2026Did you know the FDA has a secret database of all the stability failures they don't tell you about? The 27 warning letters? That's just the tip. There's a whole underground network of labs that get quietly shut down. And the new Q1F draft? It's not for biologics. It's for covering up the fact that they've been lying about shelf life since 2012.
Bailey Adkison
January 5 2026The 12-month wait is a tax on innovation. If you're smart you start testing before you even finish synthesis. But the system punishes speed. They want slow. They want paper. They want control. Meanwhile, people are dying waiting for new cancer drugs because someone in a lab in Switzerland had a power outage.
Michael Dillon
January 6 2026I've worked with 12 different CROs. Every single one of them says the same thing: 'We follow the guidelines.' But none of them can tell you why 60% RH is magic. It's not science. It's tradition. And tradition is just laziness dressed up in a lab coat.
Sophie Stallkind
January 7 2026The precision required in stability testing is not merely procedural-it is a moral obligation. Each data point represents a patient's life. The adherence to ICH standards, while cumbersome, ensures that the integrity of pharmaceuticals is preserved across diverse environments. The cost of failure is measured not in dollars, but in trust.
Katherine Blumhardt
January 8 2026i think we need to just use ai to predict all this stuff and stop wasting money on rooms with thermometers lol like why are we still doing this in 2025??